Atoms are important because they form the basic building blocks of all visible matter in the universe. There are 92 types of atoms that exist in nature, and other types of atoms can be made in the lab. The different types of atoms are called elements. Most elements have differing numbers of neutrons among different atoms, with these variants being referred to as isotopes. For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent have seven neutrons, and a very small fraction have eight neutrons.
Atom Explain
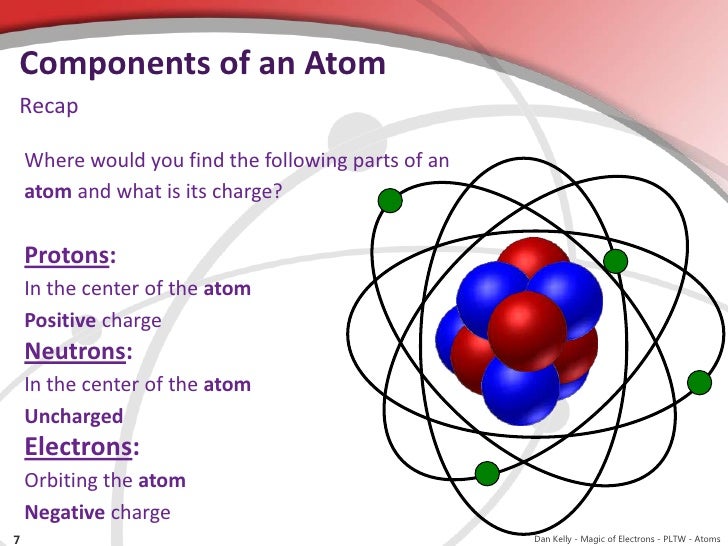
What is an atom?
Familiar and 'ordinary' matter, like the wood in a desk, the meat on your bones, the stones in your garden, as well as the
Atoms make up elements
Hydrogen is what we call an element. Iron, carbon, oxygen, sulphur and uranium are all elements. There are currently
their atoms collapse and decay almost as soon as you detect them. Fortunately, however, enough are stable to give the 92
000 000 atoms of iron (1 x 10^25 or 1E+25 atoms).
Atoms make molecules
Atoms can stick or bond together in certain permissible combinations to form groups of atoms called molecules. In the
as what you might find in a rock shop. These rings, and other similar groups of atoms, are called molecules. Atoms of different
of atom. Water is made up of H2O molecules - molecules made up of two hydrogen (H) atoms and one oxygen (O) atom
Atoms are made up of vibrations or waves
Think of a water wave on the sea. You would be right in thinking that a wave is a repeating or periodic oscillation or vibration,
vibrations. Atoms are no exception, so we need to say a little about waves first. Consider a simple wave, either a water wave
Familiar and 'ordinary' matter, like the wood in a desk, the meat on your bones, the stones in your garden, as well as the
Atoms make up elements
Hydrogen is what we call an element. Iron, carbon, oxygen, sulphur and uranium are all elements. There are currently
their atoms collapse and decay almost as soon as you detect them. Fortunately, however, enough are stable to give the 92
000 000 atoms of iron (1 x 10^25 or 1E+25 atoms).
Atoms make molecules
Atoms can stick or bond together in certain permissible combinations to form groups of atoms called molecules. In the
as what you might find in a rock shop. These rings, and other similar groups of atoms, are called molecules. Atoms of different
of atom. Water is made up of H2O molecules - molecules made up of two hydrogen (H) atoms and one oxygen (O) atom
Atoms are made up of vibrations or waves
Think of a water wave on the sea. You would be right in thinking that a wave is a repeating or periodic oscillation or vibration,
vibrations. Atoms are no exception, so we need to say a little about waves first. Consider a simple wave, either a water wave

